The near-term impact: Intersection of QC, AI and classical computing
The most promising near-term advancement is combining QC with AI and classical computing in hybrid workflows. This combination leverages the strengths of all technologies, enabling more accurate simulations of complex systems, enhanced machine learning models and improved process optimization for larger datasets at significantly faster speeds. More than 70% of biopharma stakeholders anticipate that QC will augment classical computing and AI, offering more precise and efficient solutions, especially in navigating breakthroughs in drug discovery and development.
For example, Qubit Pharmaceuticals leverages QC for advanced target characterization and molecular dynamics within small-molecule drug discovery while simultaneously utilizing AI-driven generative modeling, virtual screening and predictive analytics. Additionally, Qubit has partnered with Pasqal to leverage both classical computing and QC to model proteins, NMEs and water molecules at high levels of accuracy.
Further, IonQ’s collaboration with AstraZeneca includes the creation of an applications development center within AstraZeneca’s BioVentureHub to advance QC for drug discovery and development. In addition, IonQ has collaborated with NVIDIA, AstraZeneca and AWS to advance drug development using computational tools — achieving 20x speedups in molecular simulations versus AWS’ previous implementation — and paving the way for quantum-accelerated biopharma and materials science.
Further advancements, including running AI on quantum computers, are exciting but not expected to be seen for longer periods of time.
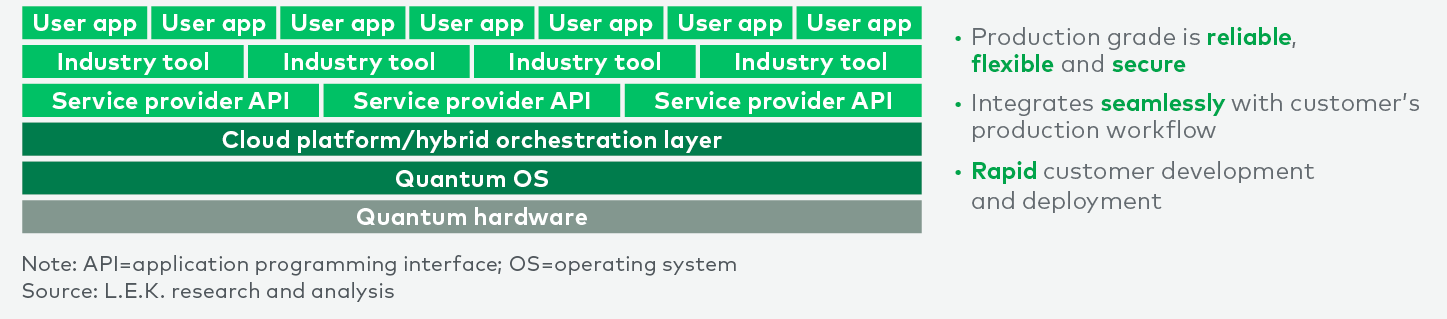
The path forward for QC in biopharma
The integration of QC into the pharmaceutical industry holds immense potential to revolutionize drug discovery and clinical trials. While QC represents a longer-term (five-to-10-year) strategic investment requiring scalable hardware, advanced error mitigation and correction, and specialized algorithms, the opportunities it presents are significant. QC can enhance predictive analytics, optimize clinical trial designs and expedite the discovery of novel therapies, ultimately accelerating drug development and reducing time to market for new treatments.
Despite current challenges such as talent acquisition and a steep learning curve, strategic investments, partnerships and AI integration can enable the industry to harness QC’s transformative power. Continued collaboration and innovation will be crucial.
Biopharma stakeholders should address the following key questions to effectively utilize QC’s benefits and remain competitive:
- Does my organization have a clear plan on how to experiment with and deploy QC within key functions, especially R&D?
- Within R&D, are there specific use cases that would be most appropriate for QC? On what basis should these be identified?
- How do I balance external partnerships and collaborations alongside internal capabilities to accelerate realization of the potential from QC in R&D?
- To implement QC effectively, what key internal operating model requirements must be met, specifically regarding talent, hardware, data infrastructure and software?
- To what extent should QC be leveraged alongside AI? Is there a benefit from integrating early (e.g., hybrid workflows) or operating independently prior to integration? What is the optimum roadmap for my organization?
By considering these questions and investing strategically in QC, the pharmaceutical industry can harness new opportunities and achieve remarkable progress across drug discovery, clinical development and operation, the supply chain, and manufacturing.
Note: L.E.K. conducted a number of interviews with both AI and pharma experts including Google, IONQ, Qubit and others to help triangulate and inform the findings.
For more information, please contact us.
L.E.K. Consulting is a registered trademark of L.E.K. Consulting LLC. All other products and brands mentioned in this document are properties of their respective owners. © 2025 L.E.K. Consulting LLC
Endnote
1L.E.K. analysis of Evaluate Pharma.