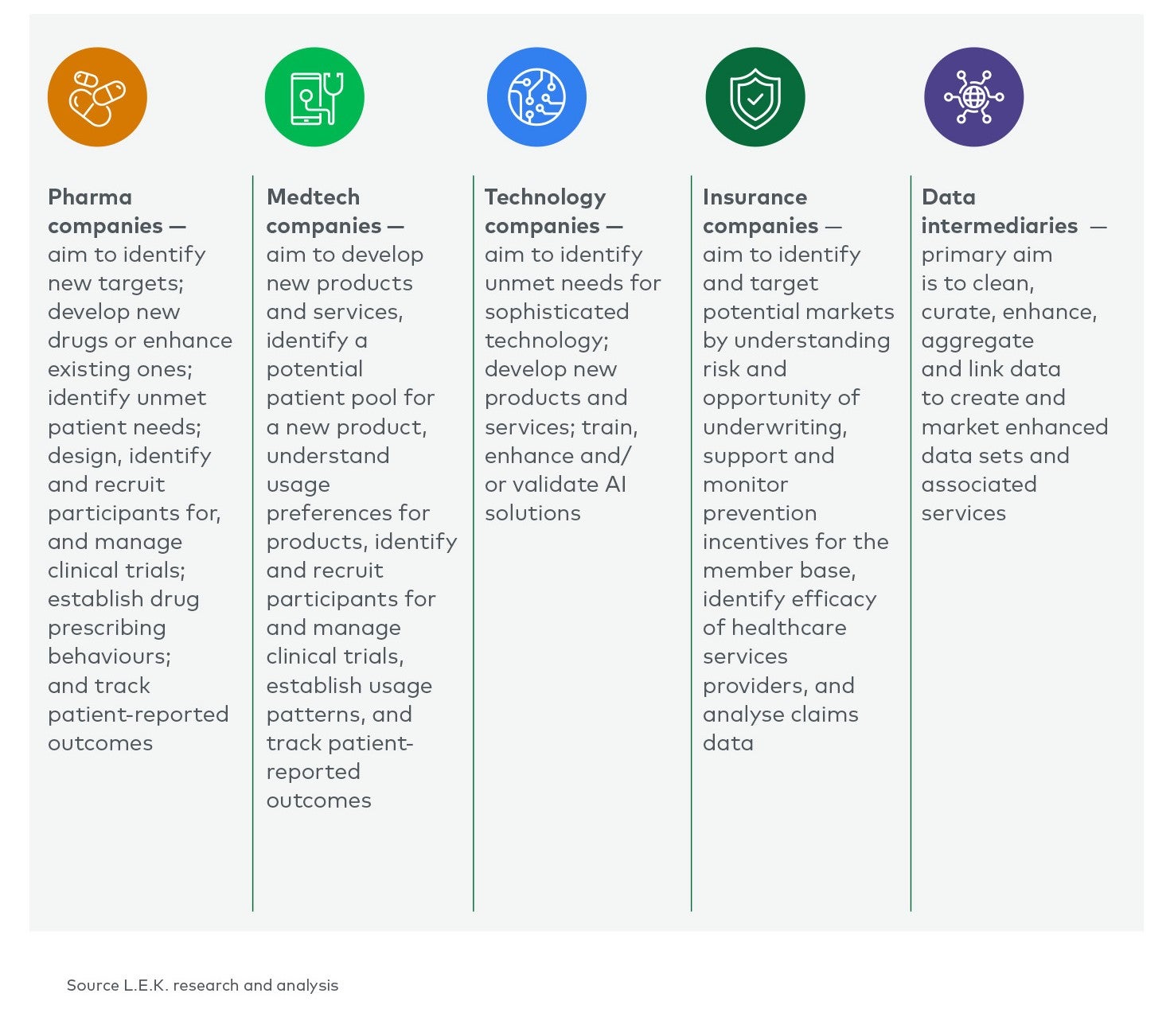
Conclusion
The implication for healthcare providers is clear - launching a data-led offering has the potential to be a profitable new business area. For impact investors in the healthcare sector, these offerings can also lead to faster new drug, medtech and/or AI development and better patient outcomes in the medium to long term.
However, there are crucial issues to consider:
-
The data set itself must be fit for purpose. A data set that is compliant with privacy and security regulations, complete (ideally longitudinal) and highly detailed, standardised to a format that is transferable across data originators and geographies, and with broad geographic coverage is best.
-
The appropriate route-to-market strategy must be carefully selected through analysis of implementation costs and monetisation profiles. Implementation costs are driven not only by the business development team, but also by a suitable data architecture that needs to be developed, a technology platform/provider that needs to be selected, and the day- to-day running of the business operations.
With knowledge and care, healthcare providers can position themselves to launch a successful new business with data-led offerings that can grow in value as new patient data is continually added to the existing base.
Adjacent companies such as healthcare IT companies providing EMRs, practice management systems or imaging software have opportunities too. They should begin developing their strategies and consider investing in and developing corresponding data platforms so that their clients are also enabled to launch these new data-driven business models and/or can better leverage data and benchmarks to enhance their own operations.
How L.E.K. Consulting can help
As data becomes an increasingly important part of the healthcare and life sciences landscape, we have extensive experience advising and supporting business leaders and their companies on their data vision and strategy, and can help develop actionable plans to deliver tangible benefits.
The authors would like to thank Amy Owens, Associate; Adorjan Gyarfas, Senior Associate Consultant; and Kunle Olaoye, Consultant, for their valuable contributions to this work.
L.E.K. thanks Sharon Lamb and Deniz Tschammler from McDermott Will & Emery for their contributions on legal analysis in this article.
L.E.K. Consulting is a registered trademark of L.E.K. Consulting. All other products and brands mentioned in this document are properties of their respective owners. © 2023 L.E.K. Consulting
Endnotes
1Genomics, transcriptomics, metagenomics, proteomics, metabolomics, inflamomics, lipidomics, glycomics, etc.
2This communication has been prepared for the general information of clients of L.E.K. Consulting. You should not rely on the contents. Although it touches on certain legal and regulatory aspects it does not constitute legal advice and should not be regarded as a substitute for legal advice or any recommendation.
3The ranges result from different percentages driven by stakeholder group — e.g. usage of non-anonymised/pseudonymised data is higher with insurance companies than with life sciences companies.
i-iiiRBC Capital Markets
ivValue range of patient clinical data was derived from both primary and secondary L.E.K. research, based on direct survey results and transactions that were valued on data volume and quality
v-viiThe 2023 L.E.K. Data Survey in Healthcare, Medtech and Life Sciences